The paediatrics department at AIIMS Delhi recently wrote a recommendation letter for the father of an 11-month-old patient who is seeking financial assistance for the infant’s treatment worth Rs 17.5 crore (USD 2.1 million) due to SMA (Spinal Muscular Atrophy) type-1.
About Spinal Muscular Atrophy) type-1:
- Spinal Muscular Atrophy is a neurological condition caused by a defect in the SMN1 gene.
- Normally, every person is born with a gene called SMN1 which produces a protein called SMN protein, in many cells in our body.
- This protein is essential for the normal functioning of nerve cells in the spinal cord called the ‘anterior horn cells.
- The anterior horn cells control the skeletal muscles essential for all our movements However, the absence of the SMN1 gene causes a reduction in the amount of SMN protein produced in anterior horn cells.
- Impacts of reduced SMN protein: The reduced quantity of SMN protein causes gradual death of anterior horn cells, and thus progressive weakness of muscles of limbs, trunk and breathing and swallowing muscles.
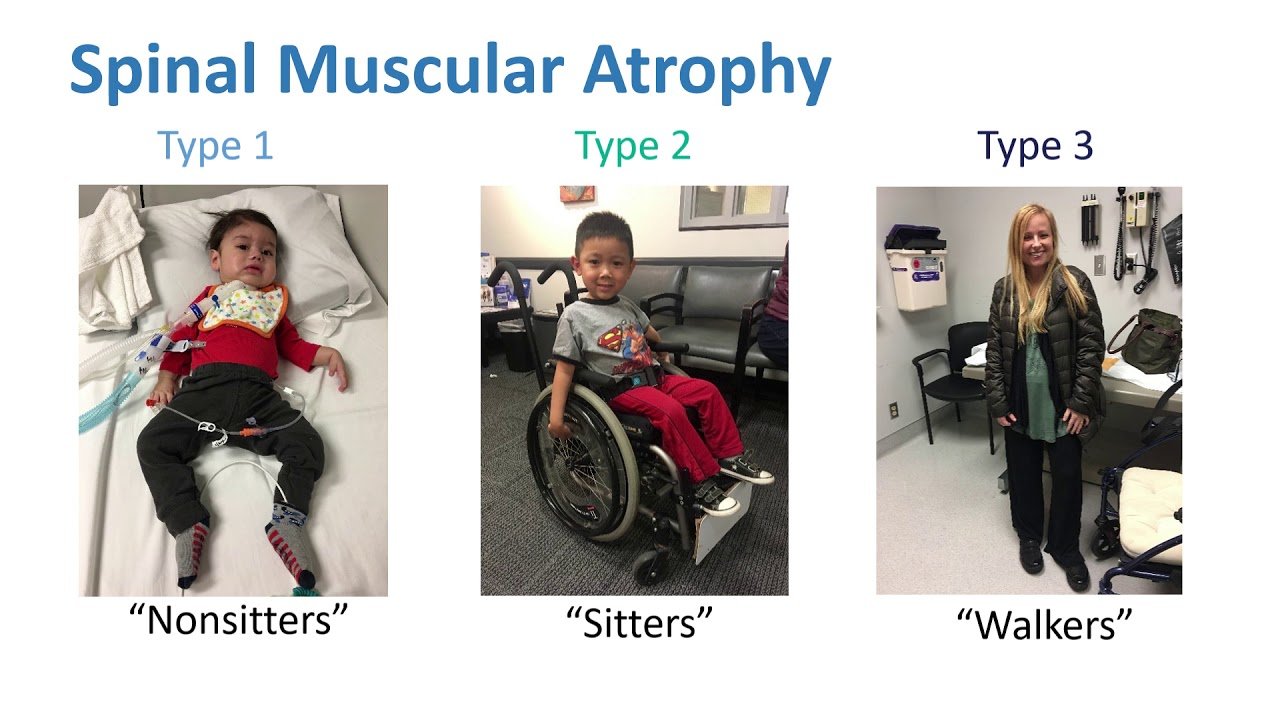
There are broadly three types of SMA
- SMA Type 1: It is the most severe type of SMA. The child will never achieve independent sitting, and at best, can attain neck holding and rollover.
- SMA Type 2: These children will attain sitting without support, but, will not be able to walk independently.
- SMA Type 3: This is the milder form of SMA. The affected persons can walk independently but have difficulty walking upstairs.
How is SMA treated?
There are currently three types of medicines available for the treatment of children and adults with SMA.
- Spinraza (Nusinersen): This is an ‘exon skipping’, with medicine injected into the spine. There is no age limit for the administration of this medicine. It works by increasing the quantity of SMN2 expression, and thus SMN protein quantity.
- Zolgensma: It is an artificially prepared SMN1 gene, coupled with an innocuous viral vector (AAV9) and administered as a single-dose intravenous infusion. This medicine is approved for use in children under two years of age
- Risdiplam: It is the most recently approved drug (in 2020). It is in the form of powder and the reconstituted solution is given orally, once daily, life-long.